On December 14, 2011 the New York Times published an article saying that three U.S. senators have proposed a bill reinforcing medical device regulations. The authors of the bill were Richard Blumenthal (D-CT), Herb Kohl (D-WI), and Charles E. Grassley (R-IA). These senators are providing bipartisan support for a “wave of medical device industry-friendly” bills that would restructure regulations of the Food and Drug Administration (FDA), requiring more stern clearances of medical products.
Besides authoring the bill, they also sent inquiries to five major manufcaturing companies of medical devices, consulting them how they track product safety and recall devices. The manufacturers were Johnson & Johnson, an artificial joint maker; Zimmer Holdings, another producer of artificial joints; Medtronic, a heart and spinal implant producer; Boston Scientific, a heart device maker, and C. R. Bard, a surgical implant maker.
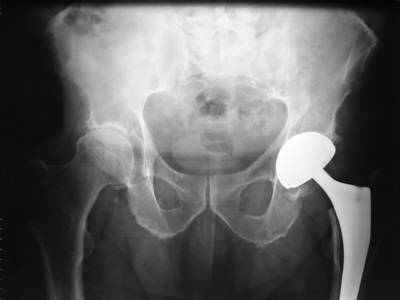
As for Johnson & Johnson’s case in particular, after informationwas out saying that the devices have defects in one out of eight clients, the ASR XL Acetabular System and the ASR Hip Resurfacing System were withdrawn. There are about 93,000 recipients of these products worldwide. Factory production and sale of the two prosthetic items started in 2003. In August 2010, DePuy carried out a worldwide recall.
Problem with Metal-on-Metal Hip Replacements
Design problems with metal-on-metal hip implants, particularly the DePuy ASR hip devices, may cause the metal components to rub against each other and shed microscopic metal particles into the body. Body reactions to these metal particles may cause soft tissue damage, cause inflammatory reactions and lead to bone loss. This may possibly result in a need for a hip revision operation, a more risky and agonizing surgery.
The presence of the metal parts may boost the amount of chromium and cobalt in the blood. This may cause metallosis (blood poisoning) and genotoxicity (genetic damage).
In a statement, a spokeswoman for DePuy, Mindy Tinsley, said, “We believe we made the appropriate decision to recall at the appropriate time given the available information.”
Johnson & Johnson Faces Legal Complaints
Johnson & Johnson have been charged with compensatory claims by their patrons, legal specialists say. They were the suffering victims of DePuy product failures and defects, among them displacements, fractures and loosening. According to legal obsevers, the DePuy hip replacement recall should stand as a lesson to other device manufacturers to strengthen and certify the safety of their products.
References:
• nytimes.com/2011/12/15/business/bill-would-require-more-monitoring-of-implants.html?_r=1
• arthritistoday.org/news/asr-depuy-hip-replacement-recall078.php
• health.ezinemark.com/patients-implanted-with-defective-mom-hip-devices-at-high-risk-of-metallosis-7d32146a00c7.html
• usrecallnews.com/2011/01/a-brief-background-of-metallosis-cobaltism-and-metal-on-metal-hip-implants.html
Besides authoring the bill, they also sent inquiries to five major manufcaturing companies of medical devices, consulting them how they track product safety and recall devices. The manufacturers were Johnson & Johnson, an artificial joint maker; Zimmer Holdings, another producer of artificial joints; Medtronic, a heart and spinal implant producer; Boston Scientific, a heart device maker, and C. R. Bard, a surgical implant maker.
As for Johnson & Johnson’s case in particular, after informationwas out saying that the devices have defects in one out of eight clients, the ASR XL Acetabular System and the ASR Hip Resurfacing System were withdrawn. There are about 93,000 recipients of these products worldwide. Factory production and sale of the two prosthetic items started in 2003. In August 2010, DePuy carried out a worldwide recall.
Problem with Metal-on-Metal Hip Replacements
Design problems with metal-on-metal hip implants, particularly the DePuy ASR hip devices, may cause the metal components to rub against each other and shed microscopic metal particles into the body. Body reactions to these metal particles may cause soft tissue damage, cause inflammatory reactions and lead to bone loss. This may possibly result in a need for a hip revision operation, a more risky and agonizing surgery.
The presence of the metal parts may boost the amount of chromium and cobalt in the blood. This may cause metallosis (blood poisoning) and genotoxicity (genetic damage).
In a statement, a spokeswoman for DePuy, Mindy Tinsley, said, “We believe we made the appropriate decision to recall at the appropriate time given the available information.”
Johnson & Johnson Faces Legal Complaints
Johnson & Johnson have been charged with compensatory claims by their patrons, legal specialists say. They were the suffering victims of DePuy product failures and defects, among them displacements, fractures and loosening. According to legal obsevers, the DePuy hip replacement recall should stand as a lesson to other device manufacturers to strengthen and certify the safety of their products.
References:
• nytimes.com/2011/12/15/business/bill-would-require-more-monitoring-of-implants.html?_r=1
• arthritistoday.org/news/asr-depuy-hip-replacement-recall078.php
• health.ezinemark.com/patients-implanted-with-defective-mom-hip-devices-at-high-risk-of-metallosis-7d32146a00c7.html
• usrecallnews.com/2011/01/a-brief-background-of-metallosis-cobaltism-and-metal-on-metal-hip-implants.html
 RSS Feed
RSS Feed